Print Campaign
TYSABRI
Context
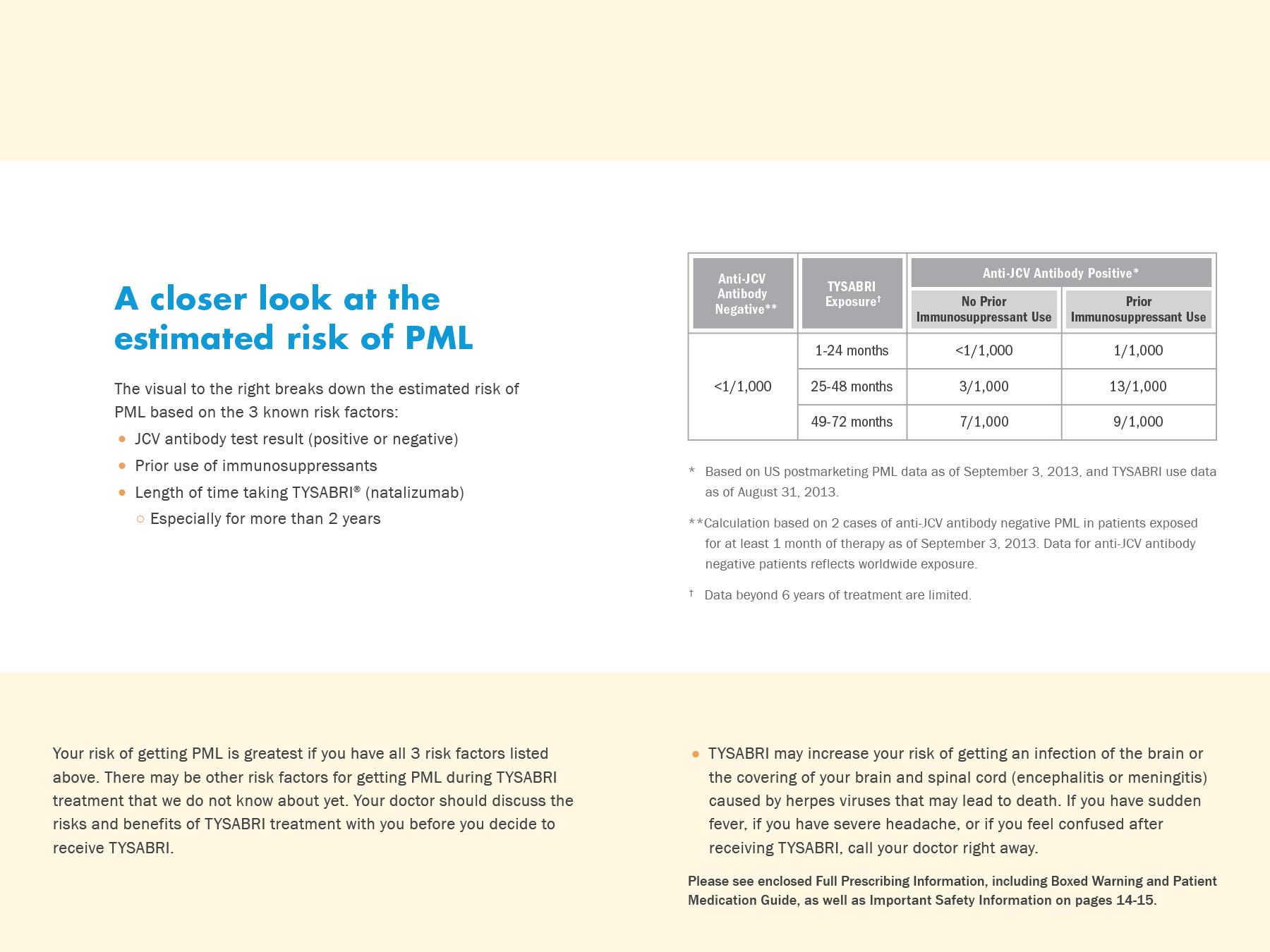
TYSABRI was pulled from the market in 2005 and then reintroduced in 2010 for restricted program usage for Multiple Sclerosis. Patients underwent extensive risk mitigation evaluation.
Goals
Win MD confidence
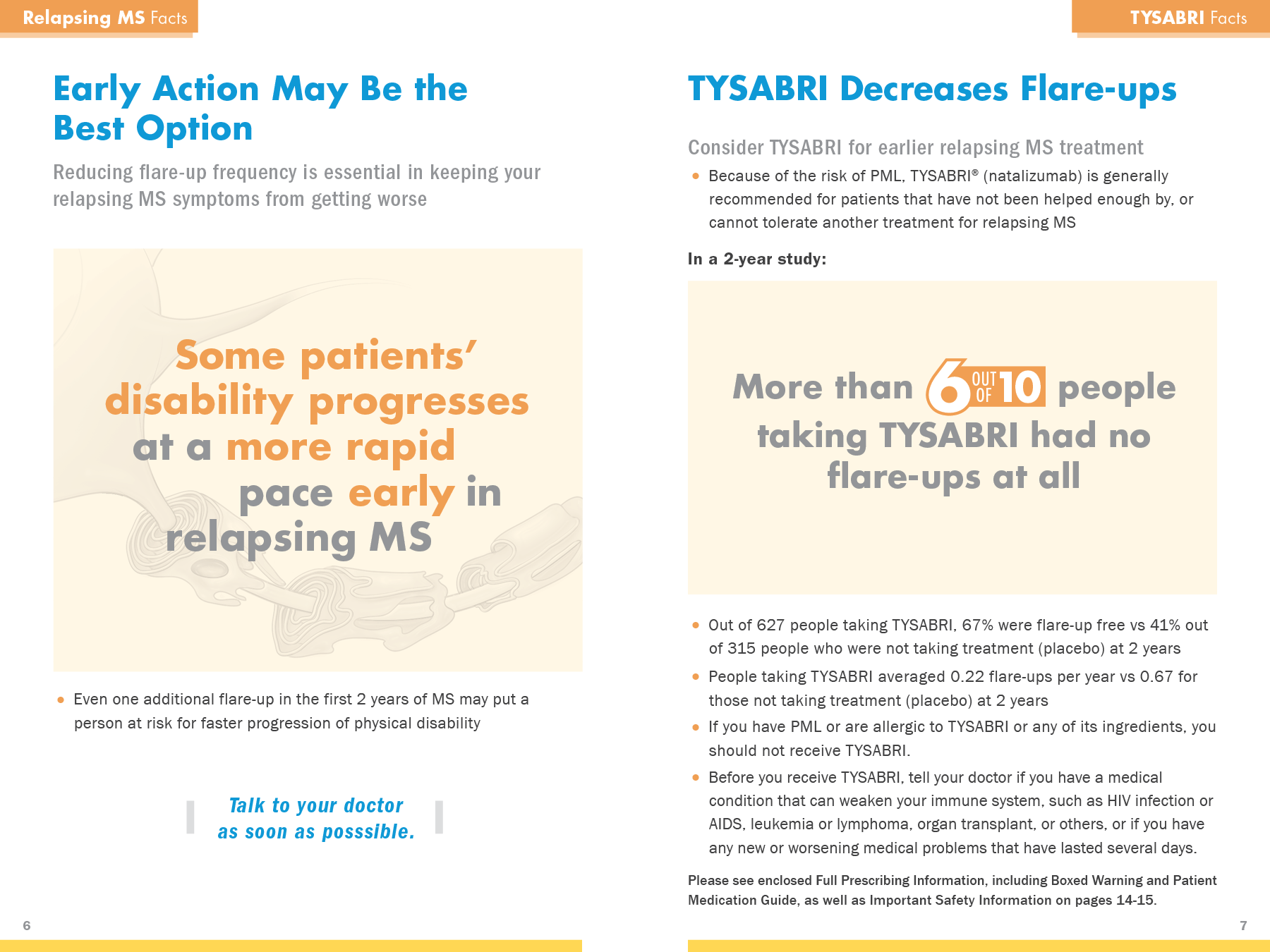
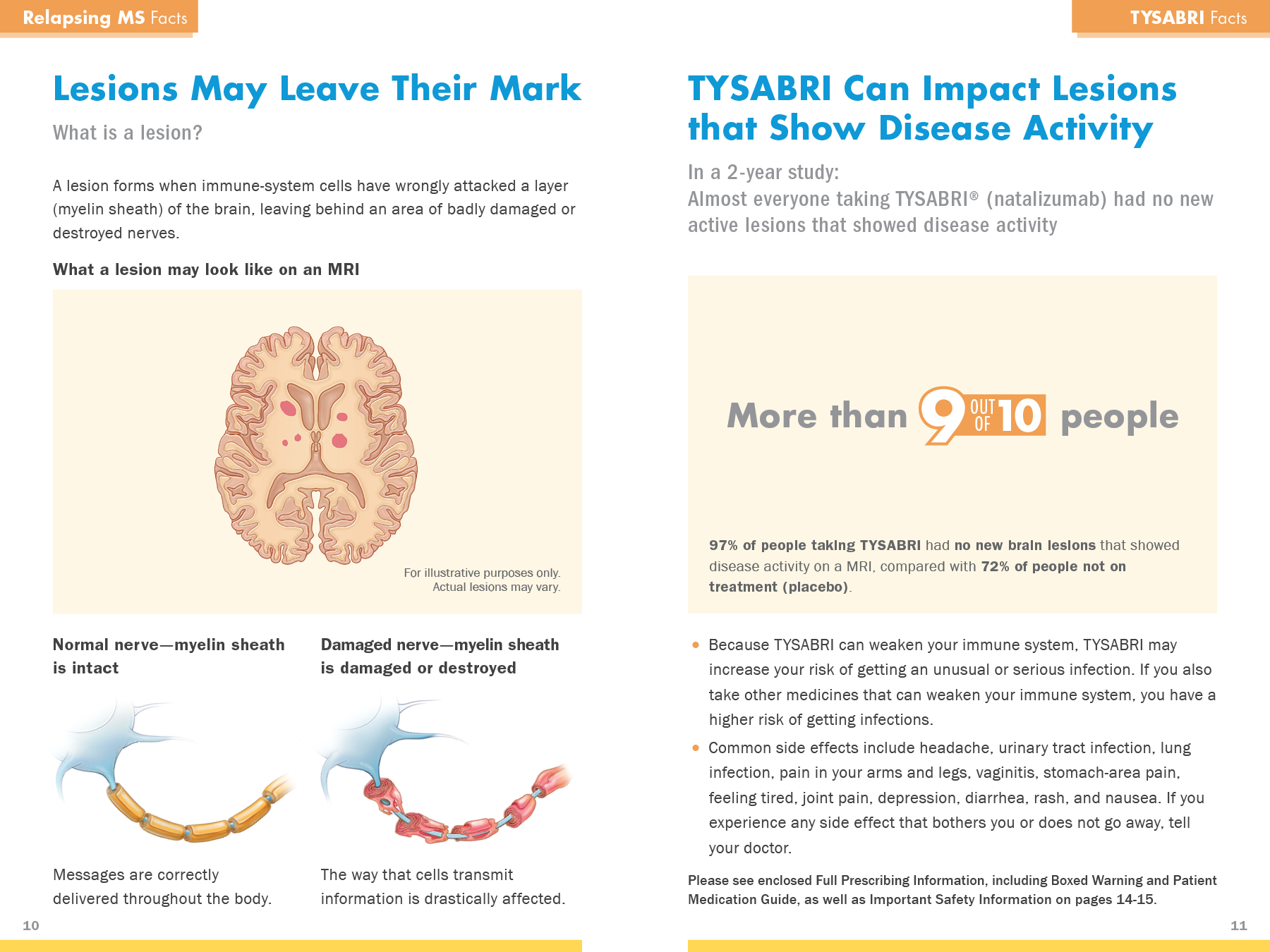
Move TYSABRI into the category of Drug of Choice for MS treatment
Preemptively address the risks and MD concerns
Educate MDs about risks and effective screening strategies
Inform patients of options – printed materials to be placed in MD offices
Results
Effective key messaging
Increased patient education
Increased prescribing of TYSABRI
Still considered a most effective option for treating MS